Is the periodic table of elements finite or are there other elements in the universe that we haven’t discovered yet? Do we have any idea what properties they might have? Could aliens make spaceships out of them?
The nucleus of each atom is made up of protons with the addition of more or less neutrons. It is thus theoretically possible for an element to exist with any number of protons in its nucleus. Perhaps a thousand or a million. But protons, as charged particles, repel each other, and the more there are in the nucleus, the harder it is to keep the nucleus together. This is partly helped by the neutrons, which act as a glue. The short-range nuclear forces between them and the protons hold the atomic nucleus together.
Even so, the larger the nucleus, the less stable it tends to be and sooner or later it will break apart. Nuclei that have half-lives of a million years or more are considered to be stable enough. The heaviest stable element found in significant quantities on Earth is uranium, with a proton number of 92. Artificially produced Curium with a proton number of 96, whose isotope 247Cm has a half-life of sixteen million years, could also be considered sufficiently stable. However, heavier elements have half-lives of years, days, and the heaviest elements of only fractions of seconds.
Moreover, the stability of the nucleus strongly depends on the number of neutrons. One isotope may be stable, but the addition or removal of a neutron turns it into a strongly unstable isotope.
The theory of magic numbers tries to explain why this is so. According to it, the nucleus of an atom is arranged like an electron shell, with energy shells that neutrons and protons fill. An atom that has one entire shell filled is more stable than others. The numbers of neutrons and protons that lead to a filled shell are called magic numbers. If an atom contains both magic numbers of protons and neutrons, it is called “doubly magic” and should be that much more stable.
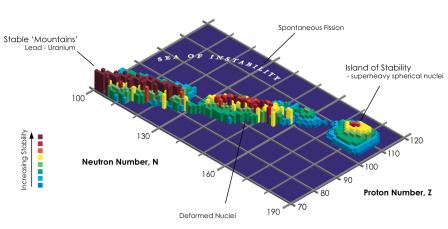
Plotting the half-lives of individual isotopes against their neutron and proton counts, we get something like a long mountain range of stability, surrounded by shallow shelves of relative stability, around which is a wide ocean of instability with isotopes with extremely short half-lives. But with a proton number above 90, the continent of stability is slowly sinking into the waters of instability.
Representation of the stability of individual isotopes as a function of the number of protons and neutrons in the nucleus. The higher the “mountain”, marked in red, the more stable the isotope. (Source: wikipedia.org)
Magic number theory has calculated that above the proton number 110 there could be a small island of stability where elements with long half-lives start to appear again. Theoretically, the most stable one should be a "doubly magic" element with a proton number of 114 and a neutron number of 184.
High atomic number isotopes are not commonly found in nature, they have to be produced in accelerators by collisions of other atoms. The heaviest atom that has been produced so far is the Oganesson. It has a proton number of 118 and its most stable isotope, 294Og, has a half-life of about 0.9 seconds. It is supposed to be a rare inert gas, but its real chemical properties could not yet be determined because only a few atoms have been produced.
The king of the stability island should theoretically be the element flerovium with proton number 114. The desired number of neutrons in the nucleus to make it stable has not yet been achieved in experiments. The closest approach is that of the isotope 289Fl (114 protons and 175 neutrons) with a half-life of about two seconds. Since the lifetime of flerovium isotopes increases with the number of neutrons, it is possible that if we can add enough neutrons, we will obtain a stable element. Theoretically, it should be a metal that is solid at room temperature, so the possibility that aliens could have made a spacecraft hull out of it is not yet completely ruled out.
Want to ask something?
Send us an e-mail with the subject “Physics mysteries” to the address:
We can't wait to tackle your interesting questions!