Hopefully, it will never be necessary. To answer your question, let’s first look at what radioactivity is, and how it is measured.
Radioactivity is the process by which unstable atoms produce ionising radiation. This radiation can also reach us from space, where it is produced by stars and dramatic processes in the cores of galaxies. Ionising radiation is divided into several types.
Alpha radiation is actually the nuclei of the helium atom. Two protons and two neutrons held together by nuclear forces. Atoms emit them when they need to change the ratio of their protons and neutrons in the nucleus to a more stable configuration. Alpha radiation can do a lot of damage in the cells of an organism, but fortunately it’s not very penetrating. It’s reliably stopped by a sheet of paper or human skin.
Beta radiations are electrons or their antiverses positrons that are produced in atomic nuclei when a neutron is converted into a proton, or vice versa, a proton into a neutron. They are lighter and can therefore penetrate material more easily. They can be stopped by a few centimetres of water or a slice of aluminium.
Processes in the nuclei of atoms are almost always accompanied by the emission of high-energy photons, so-called gamma rays. It’s highly penetrating, and a thick layer of steel or concrete will protect us from it. Photons with wavelengths between 10 nm and 100 pm are known as X-rays. They are useful in imaging the inside of the human body or metal welds.
We can also include neutrons in ionising radiation. Most often they are produced by spontaneous or controlled fission of heavy elements. They’re well shielded by a few metres of water.
All this radiation surrounds us at every moment of our lives. It’s invisible to the eye, so if we want to measure it, we have to build a detector. Many detectors are based on the ability of radiation to ionize the surrounding environment, i.e. to strip electrons from atoms, turning neutral atoms into electrically charged particles. The movement of the charged particles is an electric current that can be measured.
With sufficient electrical engineering knowledge and an equipped workroom, you could probably make a simple ionisation chamber or proportional counter. Basically, you need to detect the weak electric current that ionizing radiation induces and amplify it appropriately. But your requirement was to make something simple out of things you have in your pockets or can easily find in your surroundings.
You know the situation when, after combing your hair, the comb starts to attract small pieces of paper. A balloon rubbed with a woollen cloth behaves in the same way. By the triboelectric effect on these bodies, the charge has been separated and the comb or the balloon are now electrically charged bodies. The charge gradually escapes from them and is transferred to the surrounding bodies or the atmosphere.
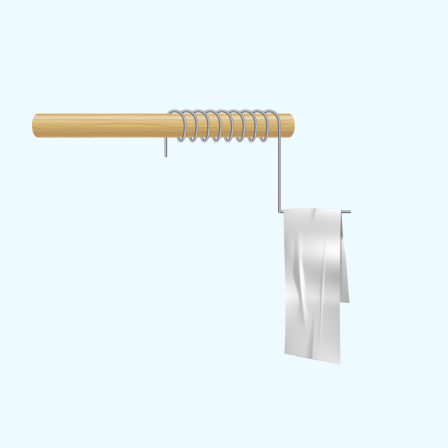
If you would like to measure how charged a balloon is, by rubbing it against a piece of cloth, you can prepare a very simple detector. Attach a piece of metal wire bent into an L-shape to a non-conductive holder, such as a piece of wood or plastic. Hang a strip of aluminium foil folded in half on the lower leg of the L. The two ends of the foil hang vertically down and close together. If a balloon is attached to the metal wire, the wire will be charged with its charge and the foil will be charged through it.
The two ends of the foil are now charged with the same charge, and as the identical charges repel each other, they lift apart. You can measure how high the foils rise and how long it takes them to fall back down.
Now you have also a radiation detector ready. Air is normally a very good insulator. If there's a lot of ionising radiation in it, its conductivity will increase. It will allow not only the conduction of electric currents, but also the discharge of electrically charged objects. If you were to bring a strong source of ionising radiation close to the charged foils, the ions produced would accelerate the dissipation of charge from the surface of the foils and the foils would come closer together more quickly; the faster, the stronger the radiation.
Unfortunately, you probably don’t have a way to test it. Common sources of ionizing radiation that you can get to and that are safe to work with, such as a radioactive piece of granite found on a walk, will probably be too weak to have a measurable effect. Stronger sources, such as the cobalt emitter from a discarded X-ray tube, are strictly not recommended to obtain, let alone work with. It could be life-threatening. Your simple radiation detector made of wire and aluminium foil will probably never measure extremely elevated radiation. Which is good.
Want to ask something?
Send us an e-mail with the subject “Physics mysteries” to the address:
We can't wait to tackle your interesting questions!